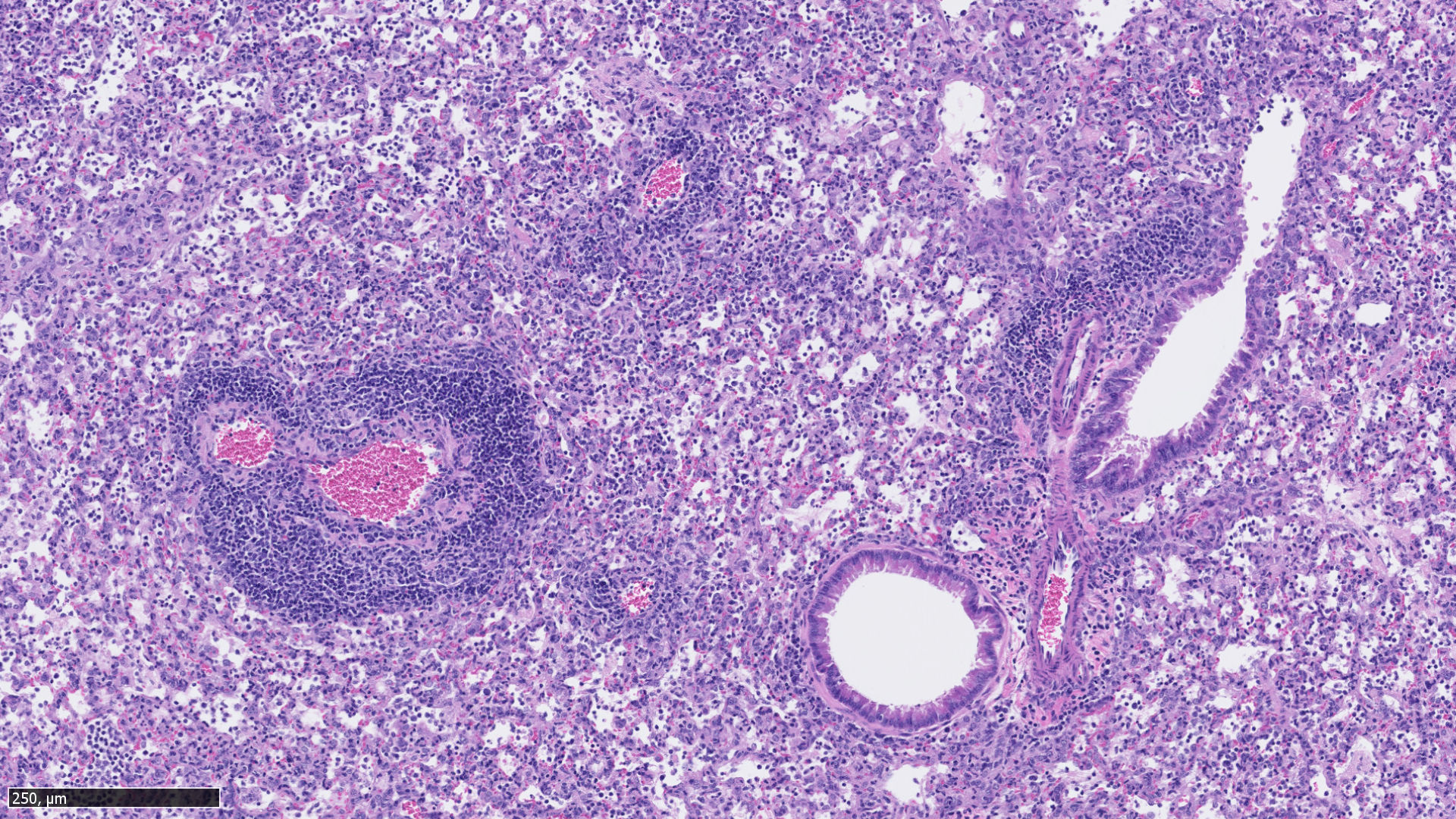
Because animal models are necessary for the development and evaluation of the immunogenicity, efficacy and safety of drug or vaccine candidates, Virnext proposes several in vivo preclinical models of influenza, SARS-CoV-2 and metapneumovirus infections dedicated to the evaluation of vaccine and therapeutic candidates. In addition to standard dose-response and adapted treatment protocols, vaccine potency assays include Hemagglutination Inhibition (HAI), MicroNeutralization (MN), enzyme-linked immunosorbent (ELISA) and immunospot (ELISpot), viral load quantification from various respiratory samples with clinical and histopathological scoring. Virnext proposes different in vivo preclinical models of infection in mouse, ferret and hamster. We propose non-human primate (NHP) models with our partner CYNBIOSE, specialized in this field.